Korean K-MASTER protocol
Funded in 2017 for 5 years with $70M by the Korean Ministry of Health and Welfare and the Ministry of Science, Information and Communication Technology,
K-MASTER aims to position Korea as a global leader of Precision Medicine based cancer treatment. This and other precision oncology efforts were the center of discussions at the
12th annual meeting of the
Korean Society of Medical Oncology in Seoul last week. Through Next Generation Sequencing based tissue panel and liquid biopsy platform for cancer diagnostics, the program will profile the cancer genome of 10,000 patients and match eligible patients to 20 pre-selected clinical trials. Ongoing trials in K-MASTER include Disease-Biomarker-Drug combinations of: CRC-MSI high-Avelumab, Any Solid-PIK3CA-Sirolimus, NSCLC-EGFR-Gifitinib, Any solid-dDDR-Nivolumab, Any solid-MET-Tepotinib, Any solid-High TMB-Nivolumab+Ipilimumab combo, Her2+ Breast-PIK3CA-Trastuzumab and Salivary-Her2-Nanoxel.
Clinical Utility
 |
| Dr. Tae-You Kim, SNUH, KSMO 2019 |
Just like the Master clinical protocols,
NCI-MATCH, led by the US National Cancer Institute and
TAPUR, led by the American Society of Clinical Oncology, K-MASTER is a non-randomized precision medicine based clinical trial where patients receive treatments (FDA approved for this cancer type or another) based on genetic changes found in their tumors. The primary goal of these Master protocols is to determine the percentage of patients whose tumors have a complete or partial response to treatment, meaning the tumors shrink by a measurable amount. In addition, side effects will be evaluated and documented. All these Master precision oncology protocols recruit eligible patients at multiple sites in their target geographies - for instance, the NCI-MATCH has 1100 sites in the US and Puerto Rico, TAPUR has 115 sites across the US and K-MASTER recruits eligible patients in Seoul Metropolitan area and participating hospitals in 5 regional hubs throughout South Korea. A key goal of these Master protocols with distributed recruiting sites is to keep the patient as close to their homes as possible while receiving treatment.
Clinical Decision Support
These Master protocols rely on accurate interpretation of genomic variants from patients to enable individualized therapy matching. However, knowledgebases containing such clinical interpretation of somatic cancer variants are highly disparate in content, structure and supporting evidence thus reducing efficiency while evaluating variants for relevance in clinical settings.
Metaknowledgebases that aggregate and harmonize evidence of clinical utility of gene variants found in patients are emerging. There is a great need for such collaborative, multi-institutional efforts across country borders to build structured, harmonized representations of cancer genomic variants to support these Master precision oncology protocols in their successful implementation across the globe. Our efforts in the
ClinGen Somatic Working Group are targeted to support such pre-competitive cataloging of clinical evidence of cancer gene variants so that the entire precision oncology community can benefit from these collective knowledgebases and the expert panels that guide this evidence curation.
We need a Hangul for Precision Oncology
To promote literacy among common people, the Korean king,
Sejong the Great created and promulgated the new alphabet called
Hangul in the 15th century. The modern Hangul consists of a total of only 24 letters with 14 consonant letters and 10 vowel letters, in contrast to the 10s of 1000s of characters in Classical Chinese that were used in Korea before the invention of Hangul. NGS data used in precision oncology master protocols can be very complex. Adding to the complexity are the numerous data processing algorithms including new machine learning methods that aim to predict treatment efficacy. As a community, we must focus on simplifying this data - just like what Hangul did to make language more accessible to Koreans - with a laser-focus on ease of usability for clinicians, patients and payers. Roche's
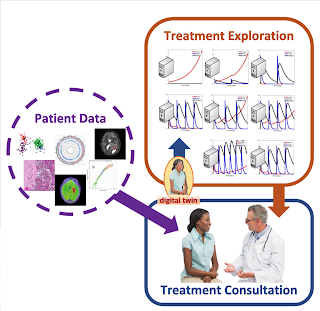
Navify Tumor Board and our own efforts in developing
asynchronous tumor boards to simplify the presentation of relevant, standardized and integrated clinical genomics information at the right time for the right patient are, hopefully, steps in the right direction to advance precision oncology based care for every patient facing a cancer diagnosis.






Comments
Post a Comment